National Institute for Healthcare Compliance
Protect Your Practice, Patients, and Reputation

WHAT WE DO
The National Institute for Healthcare Compliance (NIHC) is proud to serve as a trusted resource for up-to-date compliance information.
Our mission is to provide healthcare professionals with a centralized source of reliable and reputable guidance on regulatory compliance matters.
Please note that the materials and references provided by NIHC are intended solely for informational purposes and should not be construed as legal advice.
We aim to assist you in navigating the complexities of compliance, empowering you to make informed decisions for your practice. For specific legal or regulatory concerns, we recommend consulting with qualified legal counsel or compliance professionals.
Your Essential Compliance Guide
At the National Institute for Healthcare Compliance (NIHC), our mission is simple: to protect your practice, safeguard your patients, and give you peace of mind that you are operating fully within FDA guidelines.

Stem Cell Therapies
Red Light Therapy
Microneedling

FDA-Compliant and Non-FDA Approved Devices

General Compliance Resources
Staying informed about these resources will help ensure that your practice remains compliant with current regulations and provides safe, effective treatments to your patients.
Overview of LLLT Devices
The FDA broadly defines low-level light therapy (LLLT) devices, also known as photobiomodulation (PBM) devices, as the application of light at an irradiance that does not induce heating with the goal of altering biological activity.
See, e.g., Warning Letter CMS #612354
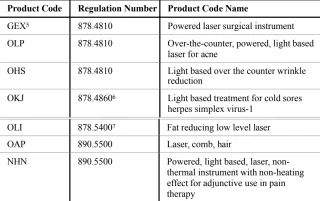
The LLLT devices currently recognized are regulated under Sections 21 C.F.R. 878.4810, 878.4860, 878.5400, and 890.5500. The product codes related to those regulations are listed in the table below.
LLLT devices that comply with the registration, pre-market clearance, and import requirements are Lawful LLLT Devices. More Information Here:
Malpractice Litigation
The discussion below can be extended to malpractice litigation by patients treated with an Unlawful Medical Device. Click Here
False Claim Act Risks
If a provider bills State and Federal healthcare programs for Unlawful Medical Devices, there is a risk of prosecution for False Claim Act violations. Providers may be entangled in their suppliers' FCA investigations even if they do not bill healthcare programs. This would require hiring appropriate legal defense counsel and time 7 away from their practice to provide testimony and/or evidence. Import Alert
Examples of unapproved LLLT Devices
In preparing this letter, I reviewed the following online listings:
• CuraYou
• LZR UltraBright
• Inlight
• NeuroCare Pro
CuraYou offers the “Neuropath Light Therapy System” on its website. This device is 9 described as using red LED light therapy and thus is an LLLT device. The website states the device will “relieve neuropathic pain” and “heals foot neuropathy,” among numerous other claims. The use of red LED light for neuropathic pain will require FDA clearance, which the company claims to have. However, searching “CuraYou” and “Leva Health” produced no results in the FDA’s medical device registration 10 database, which captures all registered companies regardless of device 11 classification. It is possible the registration has lapsed, the device is cleared under the name of a contract manufacturer, or some other circumstances explain the failure to appear in the FDA’s database. Until otherwise established and verified, it would be prudent to treat this device as an unapproved LLLT device that poses a risk to purchase or use. In contrast, NeuroCare Pro claims FDA registration but not 510(k) clearance. A search for the company produced an entry in the FDA’s medical device registration .
Navigating Stem Cells, Red Light Therapy, Microneedling, and FDA Regulations
Stem Cell Therapies
-FDA's Framework for Regenerative Medicine Products: This resource outlines the regulatory considerations for human cells, tissues, and cellular and tissue-based products (HCT/Ps), including stem cell therapies. 'Click Here'
-FDANIH Perspective on Stem Cell-Based Therapies: Provides context on the current state of stem cell therapies and emphasizes the importance of FDA approval for treatments. National Institutes of Health
Microneedling
FDA-Compliant and Non-FDA Approved Devices
General Compliance Resources

